wiesiek.euPi3cl2british swingers sitesbritish swingers stockingsbritish swingers storiesbritish swingers talkingbritish swingers tapesbritish swingers threesomebritish swingers tillybritish swingers tubebritish swingers tubesbritish swingers tumblr |
wiesiek.eu
edgenuity bot script
chris marez wheatland ca
logitech brio video flickering
student exploration weathering activity b
fusion 360 fonts
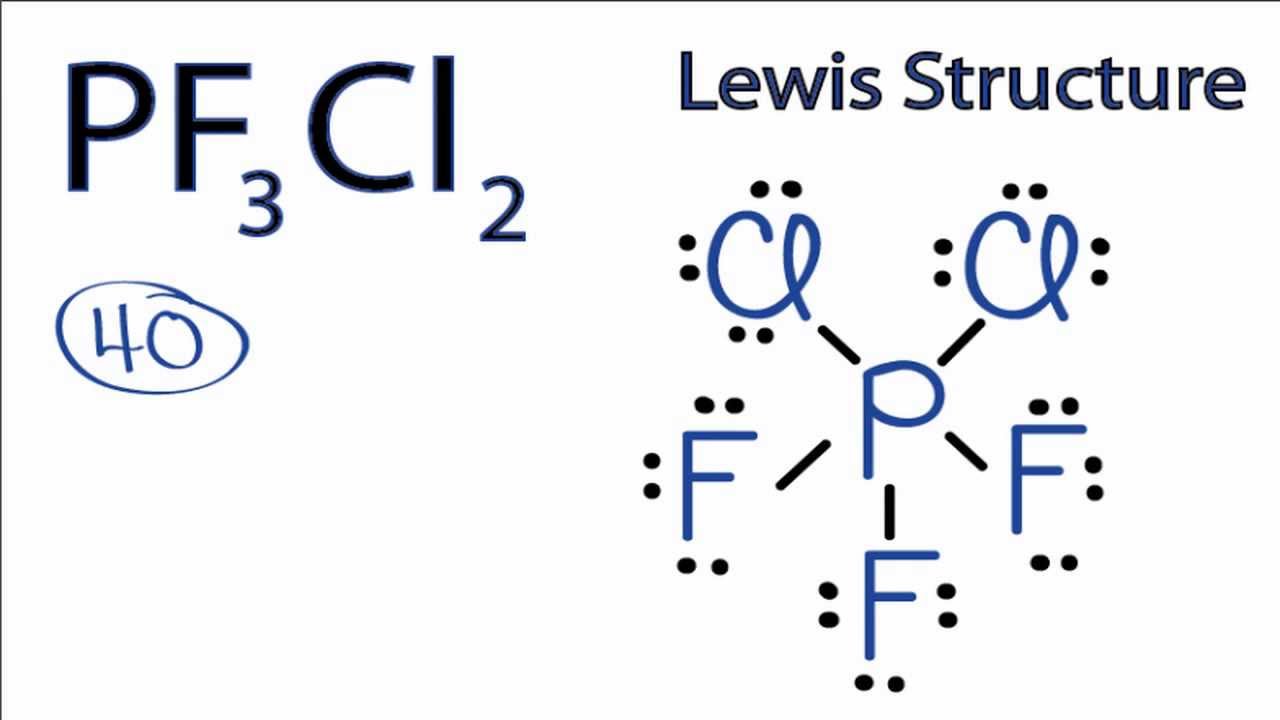
Pi3Cl2, also known as phosphorus trichloride, is a chemical compound that has gained significant attention in the scientific community due to its unique properties and applications. In this article, we will explore the various aspects of pi3cl2, from its chemical structure and properties to its uses in different industries. Chemical Structure and Properties of Pi3Cl2: Pi3Cl2 is composed of one phosphorus (P) atom and two chlorine (Cl) atoms. It is an inorganic compound that belongs to the group of phosphorus halides. Its molecular formula is PCl3, where the phosphorus atom is bonded to three chlorine atoms. Pi3Cl2 is a colorless, volatile liquid with a pungent odor. It has a molecular weight of 137.33 g/mol and a boiling point of 76.1 °C. The compound is highly reactive and can readily hydrolyze in the presence of water. Uses of Pi3Cl2: 1. Chemical Synthesis: Pi3Cl2 is widely used as a reagent in various chemical reactions. It serves as a source of phosphorus in the synthesis of organophosphorus compounds, such as phosphine derivatives. It can also be used as a chlorinating agent in the conversion of alcohols to alkyl chlorides. 2. Pharmaceutical Industry: Pi3Cl2 finds application in the pharmaceutical industry for the synthesis of various active pharmaceutical ingredients (APIs). It can be used to introduce phosphorus atoms into organic molecules, thereby enhancing their biological activity and therapeutic potential. 3. Agriculture: Pi3Cl2 is utilized in the production of pesticides and herbicides. It can be used as a precursor in the synthesis of phosphorus-based compounds, which exhibit strong insecticidal and weed-killing properties. These compounds help protect crops from pests and increase agricultural productivity. 4. Polymer Industry: Pi3Cl2 is used as a catalyst in the polymerization process. It helps in the synthesis of polymers and copolymers, which find applications in various industries, including automotive, construction, and packaging. The addition of phosphorus atoms to the polymer backbone enhances its flame retardant properties. 5. Laboratory Applications: Pi3Cl2 is commonly used in laboratories as a reagent for various chemical reactions. Its ability to react with nucleophiles makes it valuable in the synthesis of organic compounds. It can also be used for the preparation of phosphorus-based compounds, such as phosphonates and phosphates. Safety Considerations: Pi3Cl2 is a highly reactive and corrosive compound that can cause severe burns and eye damage. It should be handled with extreme caution, and appropriate protective measures should be taken when working with it. It is advisable to use it in a well-ventilated area, preferably under a fume hood, to avoid inhalation of its toxic fumes. Moreover, pi3cl2 should be stored in a cool, dry place away from sources of ignition and incompatible substances. It should be kept tightly sealed in a properly labeled container to prevent accidental spills or exposure. Conclusion: Pi3Cl2, or phosphorus trichloride, is a versatile chemical compound with numerous applications in various industries. Its unique properties make it an essential reagent for chemical synthesis and catalyst in polymerization processes. However, it is crucial to handle pi3cl2 with care due to its corrosive nature. With proper precautions, pi3cl2 can be safely utilized to enhance the efficiency and effectiveness of many chemical reactions and industrial processes. Solved PI3Cl2 is a nonpolar molecule. Based on this - Chegg. This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: PI3Cl2 is a nonpolar molecule. Based on this information, determine the I−P−I bond angle, the Cl−P−Cl bond angle, and the I−P−Cl bond angle. Enter the number of degrees of the I−P−I, Cl−P−Cl .. Solved PI3Cl2 is a nonpolar molecule pi3cl2. Based on this - Chegg pi3cl2. Chemistry questions and answers. PI3Cl2 is a nonpolar molecule pi3cl2. Based on this information, determine the I-P-I bond angle, the Cl-P-Cl bond angle, and the I-P-Cl bond angle. Enter the number of degrees of the I-P-I , Cl-P-Cl, and I-P-Cl bond angles, separated by commas (e.g., 30,45,90) pi3cl2. PI3Cl2 is a nonpolar molecule. Based on this information, determine the . pi3cl2. The bond angles in the molecule are; 180°, 120° and 90°.. The shape of a molecule is given by the Valence Shell Electron Pair Repulsion Theory(VSEPR).The number of electron domains (lone pairs and bond pairs alike) determine the shape of the molecule. pi3cl2. However, in PI3Cl2, the molecule is trigonal bipyramidal as shown. The dipoles due to the P - I and P -Cl bonds cancel out hence the .. chem chapter 9 Diagram | Quizlet. Verified answer. computer science. Add the following operation to the class stackType . void reverseStack (stackType<Type> &otherStack); This operation copies the elements of a stack in reverse order onto another stack. Consider the following statements:. PF3Cl2 Lewis Structure: How to Draw the Lewis Structure for . - YouTube. A step-by-step explanation of how to draw the PF3Cl2 Lewis Dot Structure.For the PF3Cl2 structure use the periodic table to find the total number of valence . pi3cl2. PI3Cl2 is a nonpolar molecule. Based on this information, determine the .. Answer to: PI3Cl2 is a nonpolar molecule. Based on this information, determine the I-P-I bond angle, the Cl-P-Cl bond angle, and the I-P-Cl bond.. Bond Angle : r/chemhelp - Reddit. Based on this information, determine the I−P−I bond angle, the Cl−P−Cl bond angle, and the I−P−Cl bond angle pi3cl2. Enter the number of degrees of the I−P−I, Cl−P−Cl, and I−P−Cl bond angles, separated by commas pi3cl2. So I drew it out and am assuming I is the equatorial so I-P-I has a bond angle of 120. Cl is the axial so Cl-P-Cl is .. What are the angles of PI3Cl2? - AnswerDatabritish swingers sites. I guess your PI3Cl2 compound would be similar to PCl5, which is trigonal bi-pyramidal. That is, 3 iodine atoms forming an equilateral triangle with P atom at the centre and the two chlorine atoms sitting above and below the plane formed by the triangle pi3cl2british swingers stockings. Based on this, I-P-I bond angle would be 120 degrees and Cl-P-Cl 180 degrees (linear) and I .. PI3Cl2 is a nonpolar molecule. Based on this information, determine the .. PI3Cl2 is a nonpolar molecule. Based on this information, determine the I−P−I bond angle, the Cl−P−Cl bond angle, and the I−P−Cl bond angle. Enter the number of degrees of the I−P−I, Cl−P−Cl, and I−P−Cl bond angles, separated by commas So I drew it out and am assuming I is the equatorial so I-P-I has a bond angle of 120 . pi3cl2. PI3Cl2 is a nonpolar molecule. Based on this information, - Kunduz. PI3Cl2 is a nonpolar molecule. Based on this information, determine the I-P-I bond angle, the CI-P-Cl bond angle, and the I-P-Cl bond angle pi3cl2. Enter the number of degrees of the I-P-I, CI-P-Cl, and I-P-Cl. Show Answer pi3cl2. Create an account pi3cl2. Get free access to expert answers.. PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram pi3cl2. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell pi3cl2. The molar mass of this compound is 137.33 g/mol. The melting point and boiling point of this compound are -93.6℃ and 76.1℃ respectively. Now there can be questions about the . pi3cl2. Determining Whether a Molecule is Polar - pearsoncmg.com. In this example,you will learn how to determine whether a molecule is polar.. PI3Br2 is a nonpolar molecule, how would you determine the . - Socratic. "∠I-P-I = 120°"; "∠Br-P-Br = 180°"; "∠I-P-Br = 90°". > "PI"_3"Br"_2 is an "AX"_5 molecule. According to VSEPR theory, it must have a trigonal bipyramidal geometry pi3cl2. There are three "equatorial" bonds in a trigonal planar arrangement and two "axial" bonds in a linear arrangement. So, how are the five halogen atoms arranged in the molecule? Since the molecule is nonpolar, the two "P-Br .. Phosphorus Trifluoride Dichloride, PF3Cl2 Molecular Geometry & Polaritybritish swingers stories. Electron geometry: trigonal bipyramidal. Hybridization: sp 3 d. the fluorine atoms are more electronegative than chlorine and will repel each other more strongly. Click and drag the molecle to rotate it. The molecular geometry of PF 3 Cl 2 is trigonal bipyramidal with asymmetric charge distribution on the central atom. Therefore PF3Cl2 is polar.british swingers talking. Diphosphorus Trichloride P2Cl3 Molecular Weight -- EndMemo. Diphosphorus Trichloride P2Cl3 Molar Mass, Molecular Weight
british swingers tapes. The molecule is always polar. The molecule is always nonpolar. Depending on the arrangement of. Posted one year ago. Q: PI3Cl2 is a nonpolar moleculebritish swingers threesome. Based on this information, determine the I−P−I bond angle, the Cl−P−Cl bond angle, and the I−P−Cl bond angle. Enter the number of degrees of the I−P−I,. Posted one month ago.british swingers tilly. OneClass: PI3Cl2 is a nonpolar molecule. Based on this information .. Get the detailed answer: PI3Cl2 is a nonpolar molecule. Based on this information, determinethe I-P-I bond angle, the Cl-P-Cl bond angle, and the I-P-Cl bo 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → .
british swingers tube. (Get Answer) - PI3Cl2 is a nonpolar molecule
british swingers tubes. Request PDF | Bonding in PF2Cl, PF3Cl, and PF4Cl: Insight into isomerism and apicophilicity from ab initio calculations and the recoupled pair bonding model | Following previous work on PFn and .. mastering chemistry questions unit 6 Flashcards | Quizlet. 5. Mis hermanos son _____; no tienen muchos amigosbritish swingers tumblr. 6 pi3cl2. Las gemelas tienen nueve años. Son _____. Verified answer pi3cl2. literature. Dialect refers to a variety of speech that differs from the standard speech patterns of a given culture. Vocabulary is one element of dialect.. CuCl2 - What does CuCl2 stand for? The Free Dictionary. The methanolic solutions of Ni, Co and Cu metal salts [Ni(OAc)2.4H2O, CAdegCl2.6H2O, CuCl2.2H2O] were added in 1:2 (M:L) molar ratio to a hot stirring methanolic solution of ligand L under reflux for 45 min.. Solved PI3Cl2 is a nonpolar molecule. Based on this - Chegg pi3cl2. Final answer. PI3Cl2 is a nonpolar molecule. Based on this information, determine the I−P −I bond angle, the Cl − P− Cl bond angle, and the I−P −Cl bond angle. Enter the number of degrees of the I-P-I, Cl- −Cl, and I− P− Cl bond angles, separated by commas (e.9.30,45,90) View Available Hint (s). |